Amazing Facts About Wildlife
A plant that traps animals by luring them on to a slippery surface has inspired new materials, says S.Ananthanarayanan.
The Venus Fly Trap is a well known, insect eating plant. Its leaves are divided into 2 lobes that are hinged along the midriff. Sensitive hairs on the lobes sense the entry of an insect and with the same mechanism as in nerve action, they cause the midriff
to collapse and the leaves snap shut. Another kind of trap used by plants is of the butterwort family, which has sticky surfaces, like flypaper. Once an insect is caught, the leaf may roll up or form a depression to secure and digest the prey. The bladderworts
change the salt concentration inside cavities, to force water to flow out, creating a vacuum. Trigger hairs that respond to prey open a doorway through which the animal is sucked by the vacuum, to be digested within the bladder. The corkscrew plant uses a
lobster-pot trap – inward pointing spikes that allow entry but restrict exit!

But a common variety of carnivores is the ‘pitfall trap’ kind also called pitcher plants– the edges of leaves are connected, to form a cone, or a pitcher, and the insides are made slippery, so that an animal that falls in cannot clamber out. The openings secrete
nectar, to lure the animals and the nectar has evolved to contain the alkaloid, coniine, a component of hemlock, which makes the prey lose its footing! These plants routinely trap animals as large as mice or frogs. The nature of the slippery surface, which
can shrug off water and dirt, as efficiently as the oily surface of an animal’s feet, has been of interest to create industrial materials. Tak Sing Wong and others at Harvard University report in the journal Nature that the surface of Nepenthes, and insect
eating pitcher plant, has helped them develop a synthetic, slithery material that is robust and versatile.
The Lotus effect
Lotus leaves and petals are celebrated for their water repelling quality, which keeps them clean and fresh even in grimy water. As water does not wet the lotus material, the water grabs any dirt on the surface and rolls off, leaving the surface clean and dry.
The reason for the lotus effect, then, is that water does not adhere to the surface and the droplets, to keep their surface area to the minimum, take on spherical shape. Whether a liquid adheres to a material or not depends largely on atomic composition and
whether the atoms of the liquid attach to the surface more than they attract to themselves. The angle that a drop of liquid makes at the point of contact with the surface is an indicator. The picture shows the angle of contact in a drop of water on an oily
surface, which water does not wet, and on a sheet of glass, which water does wet.
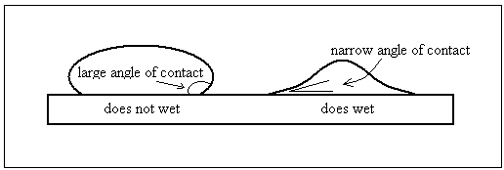
When the liquid does not wet the surface, it tries to compress itself into a ball. But in the other case, it tries to maximize the surface in contact, by spreading out. In very small drops, the internal attraction is strong and the drops remain spherical even
if the surface is attractive. Conversely, even when the surface is repelling, very large drops will spread out, because of the weight. Apart from atomic structure, in the case of the lotus leaf, the actual surface in contact is restricted by creating an irregular
surface through microscopic protrusions called papillae, coated with a water repelling wax. Because of the protrusions, air is trapped and only a small part of a water droplet is actually in contact with the leaf, and the droplet just does not adhere, but
runs off.
Oil repellent
Many initiatives to mimic the lotus leaf structure have been taken and there are now a number of water repellent synthetic surfaces, used for waterproofing, keeping surfaces moisture free to prevent ice formation, etc. But the limitation in being only ‘lotus
like’ is that the surfaces are not protected against wetting by oily liquids. The atomic structure of the water molecule has uneven distribution of charge, which makes for its strong tendency to cling and minimize its surface. In the case of oils, this quality
is not there and they do not readily form droplets. As organic and oil based contaminants are common, a surface that repels both water as well as oils would have wide application. Another limitation of ‘lotus like’ surfaces that have been developed is that
they buckle under pressure and can be damaged.
Along the lines followed for mimicking the lotus leaf, attempts to create oleophobic (or oil repelling) surfaces involved creating complex surface geometries to restrict the area of contact of oily liquids, but with limited success. The method used by the
Harvard group, reported in Nature, has been to follow alternate route, of the Nepenthes, pitcher plant, rather than the way of the lotus leaf.
In Nepenthes, the objective is prevent the feet of insects and small animals, which are coated with natural oils, from getting a grip on the ‘pitcher’ surface. This means the surface has to be ‘oil repelling’, so that the oil interface with the animals’ feet
‘slides’, rather than attaching to the surface. At the same time, the plants are found in areas of heavy rainfall and the surface needs to be waterproof, or water repellant too. The way Nepenthes manages all this is in a way that is different from the Lotus
leaf. In place of posing irregularities to restrict the area of contact, Nepenthes places a layer of liquid lubricant between the surface and the animal feet or oil or water droplet. The lubricant is held in place by protuberances, albeit not regular, on the
plant’s ‘pitcher’ surface, and it presents a smooth surface to the insect feet. The material is oil repellant and does not let the feet, which are coated with fats, from getting a grip. As pitcher plants are usually in high rainfall areas, there are spillways
to let out the water that collects, except at the bottom, where there are digestive juices that extract nutrients from the trapped animals.
SLIPS
The Harvard group has imitated the Nepenthes model in the form of Slippery Liquid-Infused Porous Surfaces (SLIPS), which is a synthetic surface with high ‘slip quotient’. The base of the material is a sponge-like substrate, impregnated with the lubricating
liquid. When a droplet of another liquid placed on the surface, the lubricant keeps the droplet away from the base material, acting somewhat like the air trapped in the protuberances on the lotus leaf. But the lubricant also forms a plane surface, like the
lubricant in Nepenthes and lets the drop, with no point of attractive contact, to move rapidly away. As the lubricant can be chosen to be oil repellant or water repellant, or even not compatible with both, the surface can be effectively ‘all purpose’. Such
surfaces can then be used to withstand composite liquids, part water-like a part oil-like.
In addition, unlike the surfaces constructed on the lotus leaf model, these surfaces can be built to withstand high pressures and as the medium is liquid, can even flow and repair scratches and localised damage. Further, by suitable selection of the base and
lubricant materials, the surface can have desired colour and transparency, enabling display applications, in addition to flow control of liquids or solids coated with liquid films.
With a variety of commercially available lubricants, the researchers are extending thee use of SLIPS and anticipate application in biomedical fluid handling, fuel transport, anti-fouling, anti-icing, self-cleaning windows and optical devices, and areas that
are beyond the reach of current technology
[the writer can be contacted at simplescience@gmail.com]
|